 Product Summary
Product Summary
This product was launched in the U.K. in 2003.
Aquamid™ contains about 2 - 3% of cross-linked polyacrylamide gel network and with the remaining part of 97 - 98% being water.
The molecule is not dissolved in water, but "swollen" like a sponge. The gel is in dynamic equilibrium with the surrounding tissue, but still the polymer retains its ability to hold water and remains elastic over an extended time.
Aquamid™ has been used for aesthetic correction for more than ten years in 30,000 patients in the previous Soviet Union and Europe.
Polyacrylamides have been used for many years in the United States and Europe for treatment of drinking water, and is also found in soft contact lenses, and tissue implant material.
Generic name
The sterile gel consists of sterile water bound to 2.5 % cross-linked polyacrylamide and does not contain microparticles.
How is it made?
Polyacrylamides are polymers formed from the polymerisation of acrylamide.
Is a skin test required before treatment?
No.
Is it temporary or permanent?
It is a permanent filler where the volume of the implant is given by water not from a solid product. Aquamid™ is made of 2.5% polyacrylamide and 97.5% water. The cross-linked polyacrylamide forms a real gel, which does not contain any solid microparticles. This makes it soft and uniform. It is injected under the skin where it can'`'`'`'`'`'`'`'t be seen or felt and remains in place permanently.
Being mostly water, the body accepts the gel readily and forms a thin membrane around the implant which helps to keep it in place; as the gel is very elastic it moves with all facial expressions.
Licenced status
Medical device.
Should be used by
Trained members of the medical profession only.
What can it be used to treat?
Aquamid™ can be used to treat deep facial lines (such as the nasolabial folds).
It can also be used to:
- Enhance lips
- Replace facial volume lost from age
- For facial deformities
- To treat depressed scars
- To enhance cheekbones and jawline.
Aquamid is not indicated for the treatment of fine wrinkles.
Not to be used in
Aquamid™ should not be used in or near anatomic sites with active skin disease or inflammation. It should not be used during pregnancy or lactation.
Duration of effect
The effect of the injection of Aquamid™ is permanent. As the initial swelling subsides there will be some loss of volume, i.e. there will be some return of the wrinkle, crease, scar or lip shape. For this reason 2 - 3 treatments may be necessary to obtain an optimum result.
The polyacrylamide gel will remain permanently in the skin.
Since the ageing of the skin continues, it may be necessary to carry out follow-up injections after a certain period of time, depending on a number of factors (skin structure, age, etc...).
Aquamid™ can be removed shortly after the injection if necessary. If the gel has to be removed at a later stage, a minor surgical procedure might be necessary, the success rate of which is variable.
Reported side effects
Injection of Aquamid™ causes only mild reaction in the surrounding tissue which is minimal compared with reactions to other foreign bodies.
This indicates that the polyacrylamide gel is well tolerated by the human body. There is no calcification, no cancer causing effect, no inflammation and no local allergic reaction.
Aquamid™ does not cause any allergic reaction or any other immunological effect either in animals or in humans.
Aquamid™ does not migrate.
Adverse effects are seen in relation to 1-2% of injections mostly infection which is easily treated.
Temporary reactions typically connected with injection may appear, for example reddening, pain, oedema, itching at the site of injection.
Within 1 -2 weeks after treatment 1 out of 1500 patients have a risk of developjng transient swelling and tenderness near injection sites. If not caused by infection these reactions are self limiting and will resolve in a couple of weeks.
Costs
Practitioners may charge somehwere in the region of £500 - £600 per syringe injected.
Clinical results
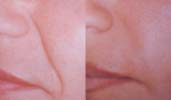
Left picture: Nasolabial folds before treatment with Aquamid.
Right picture: Improvement in nasolabial folds after treatment.
Before and after photographs are real patients, your results may differ.
Photographs provided courtesy of the Liinzi James Clinic Limited.