At the end of January 2012, following the fall-out from the PIP breast implant scandal which brought the cosmetic surgery industry to the forefront of public awareness and concerns, the Health Secretary Andrew Lansley asked the Department of Health to investigate the whole industry. This job was given to NHS Medical Director, Professor Sir Bruce Keogh along with an expert panel, and their Scope of Practice was laid down.
“I am working with experts from the plastic surgery field to look at what we can do to make sure people who choose to have cosmetic surgery and other cosmetic procedures are safe”; said Sir Keogh at the time of the announcement of the plan for a review.
He went on to say; “I will be looking at all aspects of regulation – at the regulation of implants and fillers, at whether the people who carry out cosmetic interventions have the right skills, at whether the clinics look after the care and welfare of their patients. This would include treatments such as cosmetic surgery, botox injections and dermal fillers and other cosmetic treatments where there is a potential risk to health.”
The Secretary of State for Health also requested that the review, in light of the PIP scandal, considers a national implant register, for products such as breast implants and other medical devices. The information could include the date and place of the operation, the clinical outcome as well as a method of identifying the patients who received the product – something which has been lacking in the UK for some time and which caused delays and concerns when trying to unravel the patient histories for those affected by PIPs.
As of 15th August 2012 the review, which will likely see the industry under heavier scrutiny than past Department of Health/Care Standards authority reviews, has now been officially launched with a call for evidence to include experiences and views from both the industry and patients in order to assist the inquiry. The consultation will be open for two months, ending on 15th October 2012.
The call for evidence is asking for people’s views on:
Commenting on the request for public input to the review, Sir Keogh said;
“The recent problems with PIP breast implants have shone a light on the cosmetic surgery industry. Many questions have been raised, particularly around the regulation of clinics, whether all practitioners are adequately qualified, how well people are advised when money is changing hands, aggressive marketing techniques, and what protection is available when things go wrong.”
“I am concerned that too many people do not realise how serious cosmetic surgery is and do not consider the life-long implications – and potential complications – it can have. We want to hear views from everyone, particularly people who have experience of the cosmetic surgery industry or of other cosmetic interventions – good and bad – so we can learn what works best.”
Along with Sir Keogh, an expert panel has been convened to assist in the evidence gathering and the making of recommendations to the government, based on the criteria being looked at and the results of the public consultation. The members of this expert panel are:
Despite representing a broad range of medical and lay people, nursing groups have expressed concern that their speciality is not represented, particularly given their heavy involvement in the provision of cosmetic injectables within the UK marketplace.
It’s hoped that tighter restrictions on those operating within the cosmetic surgery and non-surgical (cosmetic injectable) markets will result from the findings.
To help highlight the need for this review, the Department of Health also commission a survey which was conducted by ComRes interviewed 1,762 adults in England between 3rd and 5th August 2012 who had or who would consider having cosmetic procedures. People were asked the following questions:
The full results for both questions are here:
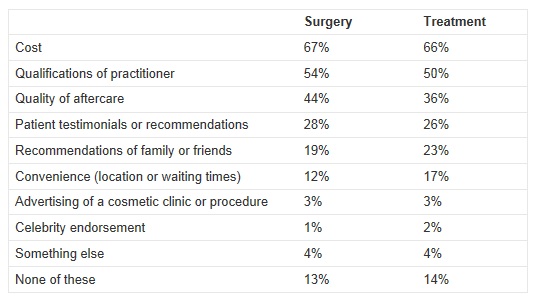
In a statement from the Royal College of Surgeons (RCS), their President, Professor Norman Williams said;
“I am concerned by the results of the survey which show that people consider the cost of the surgery more important than the qualifications of the people doing it. In an increasingly quick fix and image-conscious society, it is quite easy to forget that cosmetic surgery has lifelong implications. Patients must be assured that the practitioners have the right qualifications and level of experience. The RCS will shortly be publishing best-practice professional standards for cosmetic practice.”
Tim Goodacre, Head of Professional Standards at the British Association of Plastic, Reconstructive and Aesthetic Surgeons (BAPRAS) and a leading Consultant Plastic Surgeon, said:
“We hope that this review leads to a wholesale change of practice for the cosmetic surgery industry. We would like to see the robust implementation of a code of practice that will include higher standards of training and practice for surgeons whose work includes cosmetic procedures. We urge that institutions which deliver such operations should have the highest standard of management throughout and following interventions, and bear greater responsibility for the surgeons who work within them. We also hope that the review will address irresponsible advertising which seeks to do more than inform and instead persuade to have procedures which are widely trivialized. A compulsory code of advertising practice should be statutory and swift action taken against those who break the rules. This would protect people against mis-spelling, unrealistic expectations and the lure of cosmetic surgery ‘deals’, as well as cutting out irresponsible advertising targeted at a very vulnerable group in the population.”
“We want to ensure that people considering cosmetic surgery and procedures are given the highest standard of unbiased information, with advice and time for reflection to make an informed choice. We have some scepticism that industry can deliver such non-conflicted information, and believe there is a clear role for authoritative professional organisations such as BAPRAS and the Royal Colleges of Surgeons to support such patient information.”
“We continue to work closely with the Government to provide guidance on ensuring the highest standards of plastic surgery safety and care, including developing training, standard-setting, ongoing education, and research into better practice.”
Fazel Fatah, President of the British Association of Aesthetic Plastic Surgeons (BAAPS), said:
"We are delighted that the review is now underway. The BAAPS has been campaigning for many years for better regulations of the cosmetic surgery sector to protect patients.”
"We would like the review to take this opportunity to draw a clear line between cosmetic treatments that are seen as a commodity and cosmetic surgery that is a serious medical treatment which must be provided by fully trained and qualified surgeons.
"We would very much like the review to look at the issue of advertising of cosmetic surgery that is widely used to prey on the vulnerability of patients who seek cosmetic surgery for psychological reasons. If an outright ban is not achievable, then a new strict code of advertising is badly needed to protect patients."
The expert panel and Sir Bruce Keogh will make their recommendations to the government by March 2013.
Hey, wait!
Before you go.....
Let's stay in touch, pop your details here and we'll send our editor's hand-picked updates on your fave subjects.