Product Summary

Initially launched in the US in 2000 as Radiance FN, this dermal filler was introduced in Europe in June 2004 under the name Radiesse™.
Radiesse™ is a medical device developed by Bristol-Myers Squibb and BioForm Medical (since acquired by Merz Aesthetics) designed for the correction of small and large soft tissue deficits, but can also be use for facial shaping and contouring.
In general, calcium hydroxylapatite, the functional component of Radiesse, has been safely used in the human body for over 25 years in several indications.
Radiesse™ is becoming an increasingly popular treatment for facial aesthetics in the UK and is marketed as a volumising product.
What does it contain?
Radiesse™ is approximately 30% CaHA and 70% gel carrier by volume.
|
|
Radiesse™ is made up of synthetic Calcium Hydroxylapatite (CaHA) microspheres suspended in a gel carrier, which is an aqueous gel that contains sodiumcarboxymethylcellulose, glycerin and high purity water. The CaHA spheres are composed of calcium and phosphate ions which occur naturally in human tissue. |
It does not contain any animal or human derived components.
How is it made?
A synthetic process where calcium and phosphate ions are bonded together into calciumhydroxylapatite particles.
Is a skin test required before treatment?
No. Radiesse™ has a biocompatible formulation. The manufacturers state that it will not produce a toxic or allergic response.
Is it temporary or permanent?
Radiesse™ is not a permanent filler. It is a long lasting, resorbable filler - with the effect claimed to last approximately 2 years depending on the patient and the area treated.
Once injected into your skin, the smooth and spherical CaHA particles form a ‘scaffold’ which your own tissue grows new collagen cells between. The gel carrier dissolves within a few months and over time, the CaHA particles gradually break down and are completely metabolised by the body, leaving only the new collagen structure.
Licensed status
Medical device.
Radiesse™ is fully approved for facial augmentation with a European CE certification mark and a number of USA Food and Drug Administration (FDA) clearances.
Should be used by
Trained members of the medical profession only.

Radiesse treatment indications
Radiesse™ can be used for the treatment of:
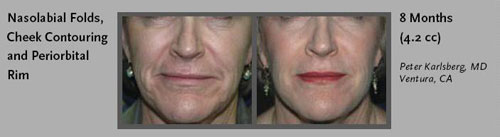
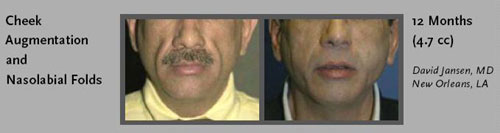
- Nasolabial Folds: between the nose and mouth
- Marionette lines: The so-called "smile lines" in corners of mouth
- Radial lip lines: lines above the upper lip
- Depressions and blemishes: like acne scars
- Shallow cheeks: caused by fat atrophy
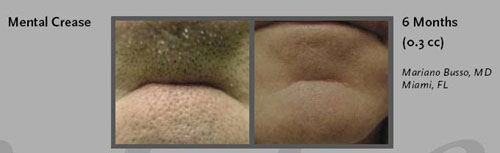
- Nose and Chin: correcting indentations, tips, bumps and unevenness.
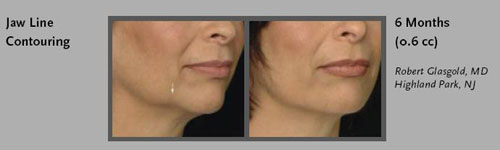
- Jaw: Improving the contours of this area
- Hand: To reduce the appearance of veins and bones in aged hands
Not to be used in
Pregnancy or lactation and people with pre-existing infections.
If you’re taking aspirin or other medications, please consult your physician.
Radiesse™ should not be injected over permanent injectable implants. If you have other implants or filler materials still in place, please notify and consult your physician.
Duration of effect
When injected in soft tissue, fibrous encapsulation of the microspheres creates volume in the treatment area.
Radiesse appears to provide a longer term result than pure Hyaluronic acid based fillers such as Restylane® or Perlane® as it is gradually replaced by the body`s own tissue, and slowly dissolves away over a 18 month or longer period.
Reported side effects
There are few reported side effects from clinical trials and actual usage data. However, some common injection-related reactions may occur, such as swelling, pain, itching, discoloration and tenderness at the injection site.
Some lumpiness at the injection site can occur, which is often due to incorrect placement of the product. The company claims that more serious granulomatous reactions have not yet been reported with the product.
Costs
Prices per treatment area vary – prices range from £150 - £450 per 1.3cc used.
Clinical results
Radiesse™has been tested extensively in clinical trials for over 8 years, with excellent safety and efficacy results.
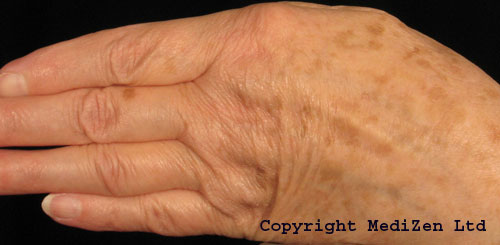
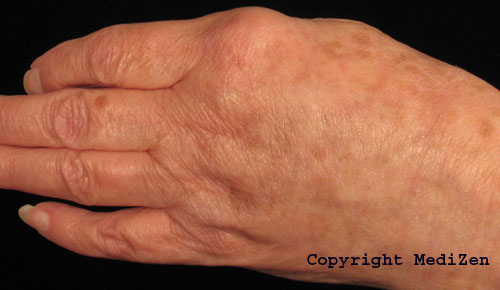
Before (top) and after hand rejuvenation with Radiesse to help reduce the appearance of veins and bones visible under thin skin on the bask of the hands.
Photographs courtesy of Annie Eccleston, MediZen Clinics Ltd
|
|
|
|
|
|
|
|
Photographs courtesy of Radiesse.
Before and after photographs are real patients, your results may differ.
Bibliography
William H. Hubbard Ph.D.
Bioform Implants
White papers
Thomas L. Tzikas MD
Evaluation of Radiesse soft tissue filler for facial soft tissue augmentation
Archives of Facial Plastic Surgery, vol. 6 July/Aug 2004
Jeffrey A. Sklar MD and Soren M. White MD
A new soft tissue filler
Dermatologic Surgery May 2004
David J. Goldberg MD, Ellen S. Marmur & Robert Phelps
Clinical histologic and electron microscopic findings after injection of a calcium hydroxylapatite filler
Journal of Cosmetic Laser Therapy 2004
Louis P. Bucky MD, Suhail K. Kanchwala MD, Lisa Holloway
Reliable Soft Tissue Augmentation – A clinical comparison of injectable soft tissue fillers for facial volume augmentation
Annals of Plastic Surgery 2005
Stephen L. Comite MD, Judy F. Liu, S. Balasubramanian, Marisa A. Christian
Treatment of HIV-associated facial lipoatrophy with Radiesse
Dermatology Online Journal, vol 10, no 2, 2004
Michael Olding MD, Karen Kim Evans MD, Yvonne Rasko BS, Joanne Lenert MD
The use of Calcium Hydroxylapatite for nipple projection after failed nipple-areolar reconstruction.
Annals of Plastic Surgery, vol 55, no 1, July 2005
Barry M. Zide MD -
Radiesse: Short term experience
Aesthetic Surgery Journal, Nov/Dec 2003
Miles H. Graivier MD -
Radiesse effective intermediate filler
Cosmetic Surgery Times, Aug 2004 & Dermatology Times April 2004