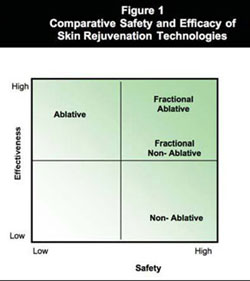
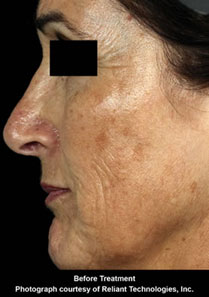
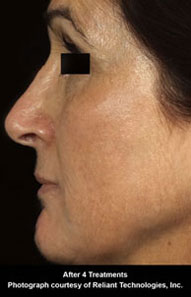
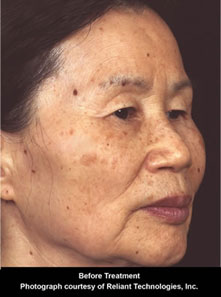
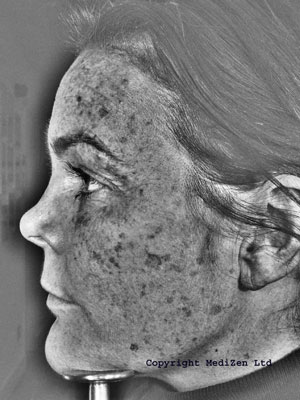
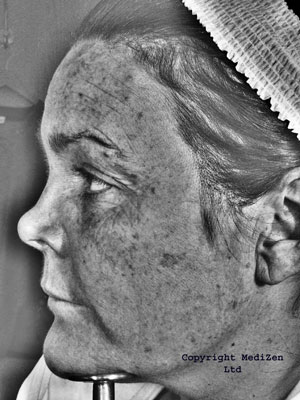
Fraxel™
The original Fraxel™ laser machine fired a 1550nm diode pumped erbium fibre laser beam which is then split into thousands of microscopic beams, producing tiny dot, or pixel-like treatment zones on the skin; referred to by the manufacturer as MicroThermal Zones (MTZs).
At 1,500 – 2,500 MTZs per cm2 (depending on machine settings), the MTZs are invisible to the naked eye, other than as an overall “bronzed” look they give to the skin. Each MTZ is about 30 - 70 microns in diameter, (a micron is one millionth of a metre), and 400 - 700 microns deep. This is in contrast with the typical depth of 200 - 400 microns achieved with ablative resurfacing; where any deeper and you risk scarring. The Fraxel™ laser treats between 12 - 20% of the skin with each treatment session.
As with ablative lasers, the Fraxel™ is also attracted and absorbed by water in the tissues; however in this case the stratum corneum, (the outermost layer of the skin, consisting of dead cells that slough off) is more or less spared from damage by its low water content, meaning it has a low absorption of laser energy.
The manufacturer, Solta Medical have since replaced their original Fraxel® SR Laser with a range of devices including the Fraxel re:fine, re:store (and re:store dual) and the re:pair.
Pixel® 2940
Launched in the US in April 2006, the FDA approved Pixel® 2940 from Alma Lasers (distributed in the UK by Advanced Beauty Cosmetics Ltd) is a 2940nm Er:YAG ablative laser delivered to the skin through a micro optics lens which creates 49 (7x7) or 81 (9x9) pixel sized ablation dots on the skin within an 11mm x 11mm treatment zone.
Alma Lasers market the Pixel® 2940 as ‘combining the proven effectiveness of an ablative approach with the patient comfort level and convenience of a non-ablative approach’.
Unlike traditional ablative lasers, used only on the face, the Pixel® 2940 is targeted at treatments on the face, neck, chest, arms and hands, giving it a wider scope for treating aged and sun damaged skin.
Lux 1540 Fractional
The Lux 1540™ Fractional device from Palomar (distributed in the UK by Eden Aesthetics) is a 1540nm erbium glass fibre non-ablative laser, which is delivered to the skin in tiny microbeams, in combination with contact cooling of the skin’s surface.
The Lux 1540™ can penetrate up to a depth of 1mm into the skin, whilst also leaving the skin’s surface intact, creating tiny columns of treated (or coagulated) tissue which are surrounded by the healthy tissue. With a choice of treatment size heads for skin resurfacing and wrinkle reduction, the Lux 1540™ can produce 100 microbeams/cm2 columns for deep coagulation (up to 1mm) with a 10mm head, or 320 microbeams/cm2 narrower columns for relatively shallow coagulation, with the 15mm head.
It was approved by the FDA in February 2007 for ‘skin resurfacing procedures and dermatological procedures requiring the coagulation of soft tissue’.
Others
Fractional lasers are a growth area within the laser technology field, with more and more companies entering the marketplace, either with new devices or modifications of existing machines. Some others you may find include:
Affirm™ from Cynosure – This is a 1440nm Nd:Yag non-ablative laser with Cynosure’s proprietary Combined Apex PulseSM (CAP) technology. This is a special lens mounted over the end of the laser that consists of approximately 1,000 diffractive elements per cm2 which put simply ‘bends’ the wavelength of light emitted from the laser so as to affect more surface area per single pulse of the laser. The Affirm™ targets the superficial layers of sun damaged skin, and delivers both a high and low energy of light within the treatment zone, due to the lens configuration, which they claim enhances collagen remodelling whilst also stimulating collagen production.
Active FX from Lumenis – This is based on the company’s existing UltraPulse Encore technology, which underwent computerised modifications, and now comprises a single treatment system for ablative fractional photothermolysis with a CO2 laser. Active FX is the most aggressive of the fractional laser devices introduced to the market so far. As such treatment with this device requires a topical anaesthetic and produces the most lengthy down time for this type of procedure.
Profractional from Sciton - This 2940nm fractional ablative laser device was launched in the US in April 2006 and in the UK in April 2007, (distributed by Sigmacon Ltd). The manufacturers claim that it differs from other fractional devices because of the method of delivery of the laser energy, which it says makes for a more comfortable treatment experience by reducing any unnecessary heat build-up. With a treatment head of 20mm x 20 mm, the Profractional can produce a variety of pattern configurations (dots) on the skin depending on the condition and area being treated. These dots can penetrate from 25 to 1500 microns in depth, depending in the settings used. The Profractional can treat from 1.5% – 60% of the skin within a treatment zone.